Who Uses the InteliSwab Rapid Test? Individuals 2 years and older, with or without symptoms.
Why They Love It: InteliSwab is incredibly easy to use. It requires only one minute of hands-on time, making it ideal for at-home, school, business, and clinical testing.
InteliSwab COVID-19 Rapid Test Overview
The InteliSwab Covid-19 Testing Kit is an antigen test that detects COVID-19 and identified coronavirus variants, including beta, alpha, delta, gamma, and omicron. It uses an all-in-one design, providing a swab and test device that offers quick and accurate results. It's one of just two COVID-19 antigen test kits that was selected by the U.S. government to facilitate testing in schools around the country.
Features and Benefits
- One Minute of Hands-On Time for Convenience
- Unique All-in-One Swab and Test Design Provides Ease of Use
- No Drops Tto Measure Reduces Any Confusing Steps
- 93% Test Accuracy in Clinical Study
- Shown in Studies To Detect Multiple Variant Types
What Comes in the Box?
- Testing device
- Developer solution vial
- Absorbent packet
- Instructions for use
- Test stand


Unique All-in-One Swab and Test Design
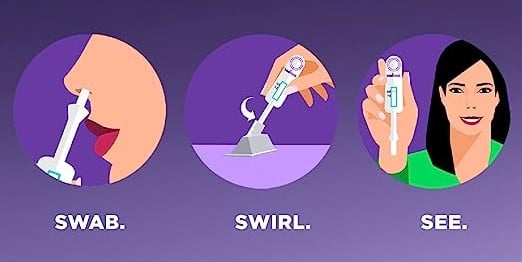
InteliSwab COVID-19 Test is unlike other rapid antigen tests because it's the only at-home testing kit that has a unique all-in-one test and swab device. The unique design allows users to easily swab, swirl, and see test results within 30 minutes, without measuring drops, connecting to smartphones, or assembling test kits.
Detectable Variants
The InteliSwab COVID-19 Test detects active proteins from the SARS-CoV-2 virus. Shown below is a list of all detectable variants:
- Beta, B.1.351
- Delta, B.1.617.2
- Alpha, B.1.1.7
- Gamma, P.1
- Omicron, BA.1, BA.2, BA.3, BA.4, BA.5, BQ.1, BQ.1.1, B.1.1.529, BA.2.12.1, XBB.1.5, XBB.1, BA.4.6

Frequently Asked Questions (FAQ)
Is this test FDA-approved and/or cleared?
The InteliSwab COVID-19 Rapid Tests did receive Emergency Use Authorizations (EUA) from the FDA, but they are not FDA-approved or cleared. The EUA declares that specific circumstances exist to justify the emergency use of in vitro diagnostics for the detection of the viruses associated with COVID-19. The EUA is supported by the Secretary of Health and Human Services. It will continue to have EUA approval until the declaration is terminated or authorization is revoked by the FDA.
Is this test reusable?
No, this is a single-use product.
Is the test easy to use?
Unlike other COVID tests, InteliSwab is incredibly easy to use. Simply swab the nostrils and swirl the applicator in the premeasured solution in the tube. After, just wait for the results. This simple method eliminates unwanted or confusing steps, such as mailing samples or using a smartphone.
Does taking this test hurt?
No. The swab is gentle and doesn't have any sharp tips. It should only be used to swab the lower part of the nostril. If any pain occurs, please discontinue use and seek help from a medical professional.
What's the difference between COVID-19 Rapid Test Pro and COVID-19 Rapid Test
The COVID-19 Rapid Test Pro is for point-of-care use by medical professionals in CLIA-waved facilities. It contains 25 tests and five stands with three vial slots, which allows healthcare professionals to run 15 samples concurrently. The InteliSwab In-Office COVID-19 Test Pro kit is ideal for batch testing, unlike the OraSure InteliSwab At-Home COVID-19 Test, which tests one sample at a time.
Product Specifications
- Manufacturer: OraSure Technologies
- Over-the-Counter Rapid Test (1001-0620)
- Quantity: Box of 24 Packs
- Tests per Pack : 2
- Rapid Test Pro (1001-0614)
- Quantity: One Kit per Box
- Tests per Kit: 25
- Test Stands: 5
- Solution Components: ProClin950 (0.1%) and Triton X-100 (0.2%)
- Unopened/Unused Stage Temperatures: 35-86 Fahrenheit
- Country of Origin: United States
- Application: COVID-19 Rapid Antigen Test


Manuals and Documents
 Instructions for Use shows how to use the test.
Instructions for Use shows how to use the test.
 Healthcare Provider Instructions for Use talks about the materials provided, warnings, handling precautions, and more.
Healthcare Provider Instructions for Use talks about the materials provided, warnings, handling precautions, and more.
 Letter of Authorization discusses the issued Emergency Use Authorization.
Letter of Authorization discusses the issued Emergency Use Authorization.



Login and Registration Form