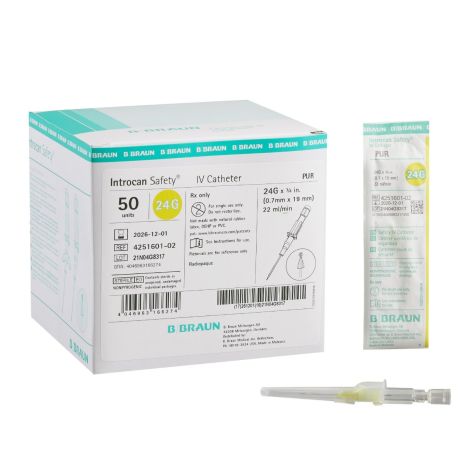
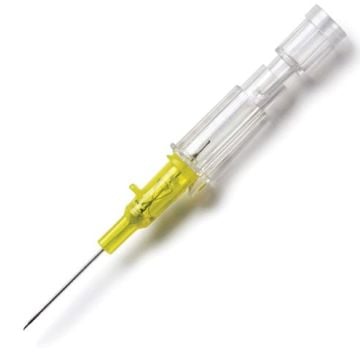
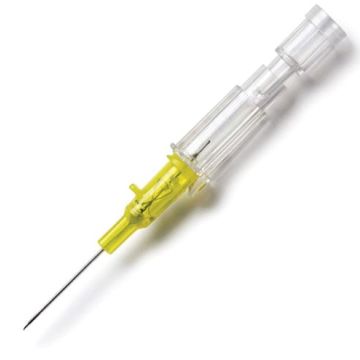
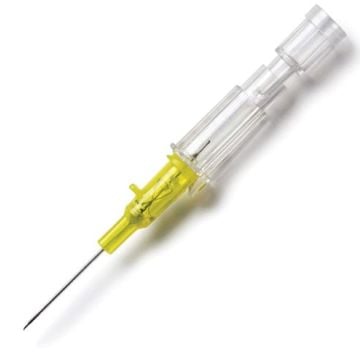
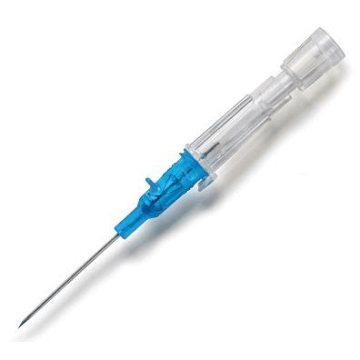
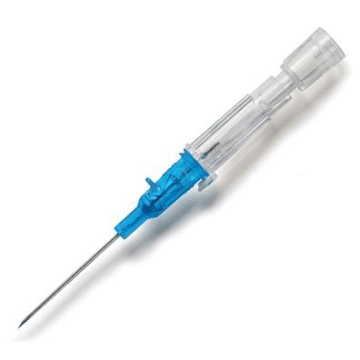
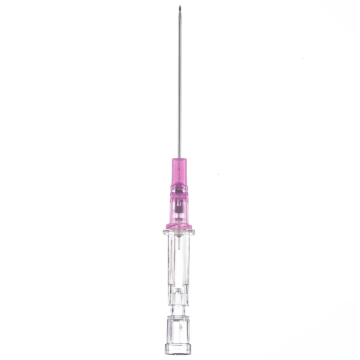
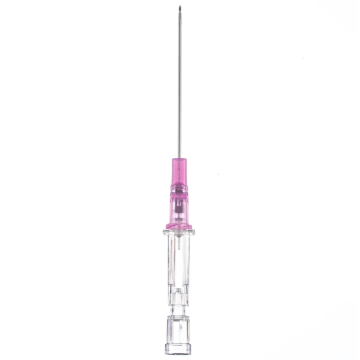
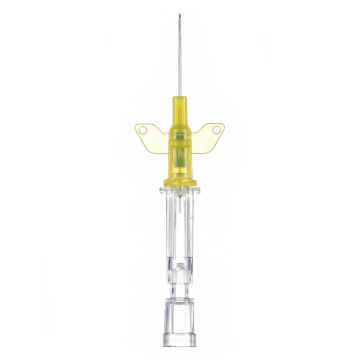
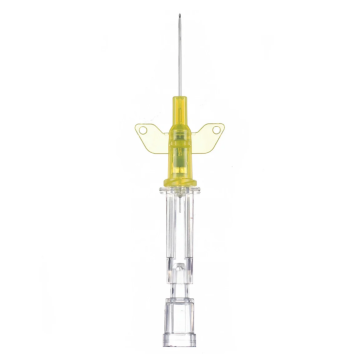
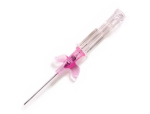
Introcan Safety IV Catheters (PUR) are made with polyurethane for softer in-dwelling performance. Braun Medical, Introcan Safety IV Catheters are available in several options to meet individual needs. PUR Catheters afford greater flexibility for a softer and more comfortable in-dwelling while FEP Catheters have a firmer construction for easier arterial access and to facilitate special procedures. Both PUR IV Catheters are available in Straight Catheters or Winged Catheter options. Each option provides a passive safety shield to protect against accidental needle sticks and facilitates one-handed operation. A built-in removable flash plug permits the attachment of a syringe for aspiration or other special procedures.
Safety IV Catheters protect caregivers and patients during the blood drawing process, including needle insertion, catheter deployment, and needle removal. The safety devices on these IV Catheters are passive, requiring no activation on the user's part. Activated automatically, the Introcan Safety IV Catheter cannot be by-passed to cause an accidental stick injury. These Safety IV Catheters have a sharp universal bevel, reducing the patient's pain. Designed with a back-cut bevel, the needle geometry offers more comfortable insertions. A back-cut incision offers a highly flexible pathway and provides easier catheter insertion with less tearing and faster healing. Introcan Safety IV Catheters save time and incur no uncomfortable safety steps found in competitor safety designs. Unlike many competitors designed Safety IV Catheters, the Introcan Safety IV Catheters guard against delayed activations or activation failures. This design also allows insertion without the high angle typically required of other needle designs. Introcan IV Catheters offers more flexibility and choice of angles for accessing difficult veins.
Introcan Safety IV Catheters Features & Benefits
- Passive safety shield requires no user activation.
- Provides safety shield to protect caregiver and patient against accidental sticks.
- Color-coded labels for easy gauge size identification.
- Minimizes needlestick injuries.
- No buttons, twists or clicks to remember.
- Locking bevel indicator easily identifies bevel-up orientation.
- Locking bevel adds stability during catheter advancement.
- Allows a choice of insertion angles for accessing difficult veins.
- Universal needle bevel provides enhanced performance.
- Removable flashplug permits syringe attachment.
- Clear flashback chamber allows easy visibility of blood entrance.
- Ribbed design provides secure handling.
- Illustrations on the box label show the contents to allow caregivers to verify the contents prior to opening packaging.
Introcan Safety IV Catheter Specifications
- Introcan Safety IV Catheter Product Numbers: 4251601-02, 4251652-02, 4251628-02, 4253523-02, 4253540-02.
- Gauge Options: 20 Gauge, 22 Gauge, 24 Gauge.
- Catheter Length Options: 0.75 Inch, 1 Inch.
- Flow Rate Options (mL per minute): 22, 35, 65.
- Hub Type: Straight or Winged.
- Needle Material: Stainless Steel.
- Needle Point: Universal Bevel.
- Safety Feature: Safety Needle.
- Application: Peripheral Venous Catheter.
- X-Ray Compatibility: Radiopaque Stripes.
- DEHP free.
- Latex free.
- PVC free.
- Sterile.
- Disposable.
- Sterilization Method: EO.
- Maximum Shelf Lift: 60 months.
- Manufacturer: B. Braun Medical Inc.
- Brand: Introcan.
- UNSPSC Code: 42221504.
- HCPCS Code: A4649.
Introcan Safety IV Catheters Additional Information
- Introcan Safety IV Catheter Brochure covers passive technology, features and product versions.
- Introcan Safety IV Catheters Use Suggestions offer step by step suggested procedure for inserting and removing IV catheters.
Braun Introcan Safety IV PUR Catheter Video (3:38 minutes)
Introcan Safety IV Catheter Steps for Using Video (7:31 minutes)






Login and Registration Form