3M Health Care is now Solventum, delivering the same trusted products under a new name.
3M Tegaderm IV Transparent Film Dressing with Border allow observation of the IV insertion site. Manufactured by 3M, are designed with a thin backing with a non-stick area around the catheter site and a non-latex, hypoallergenic adhesive border. It is designed to stabilize the catheter to minimize movement and dislodgement. This product provides a sterile barrier to external contaminants, including viruses, bacteria and liquids. The product is waterproof and has a 7 day wear time.
3M manufactures other dressings such as Transparent, including the CHG IV Securement, Foam, Hydrocolloid and Silver Alginate.
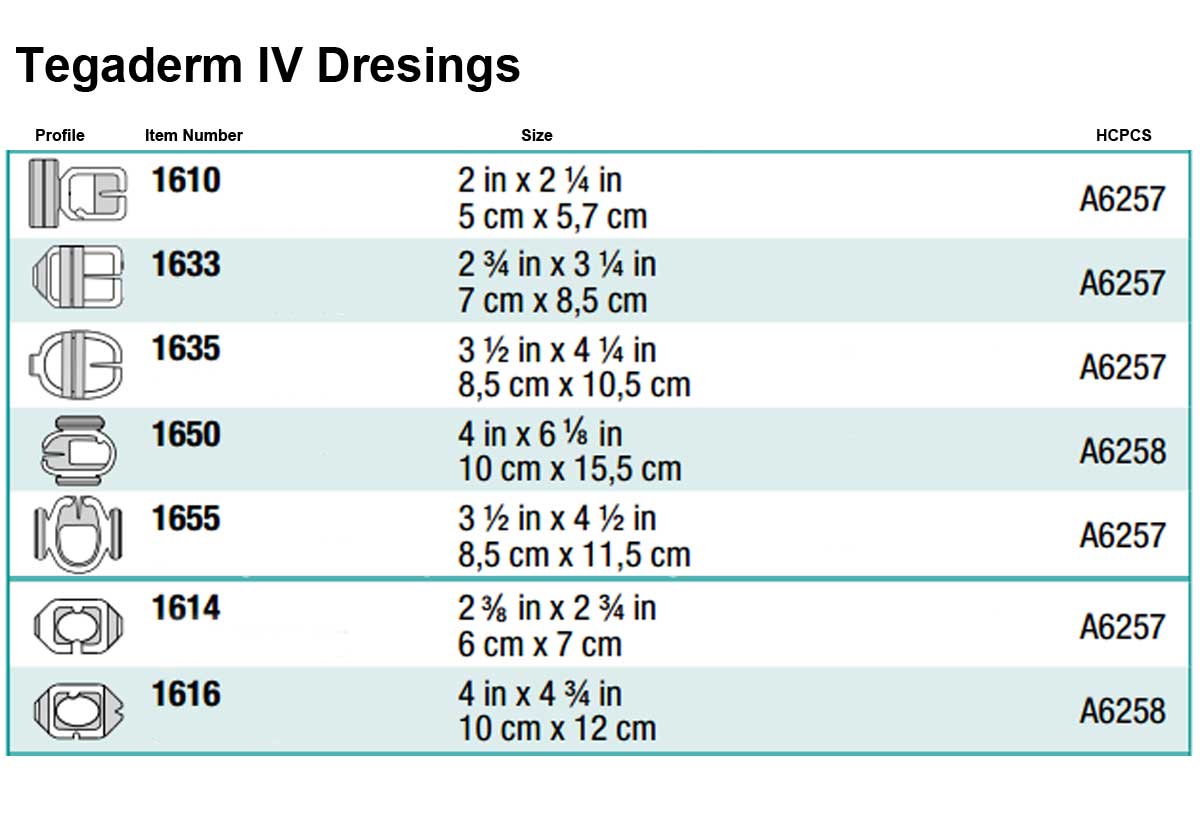
Tegaderm IV Dressings come in several different sizes and shapes. Below are three charts of the different shape options with a profile of the shape and specifications.
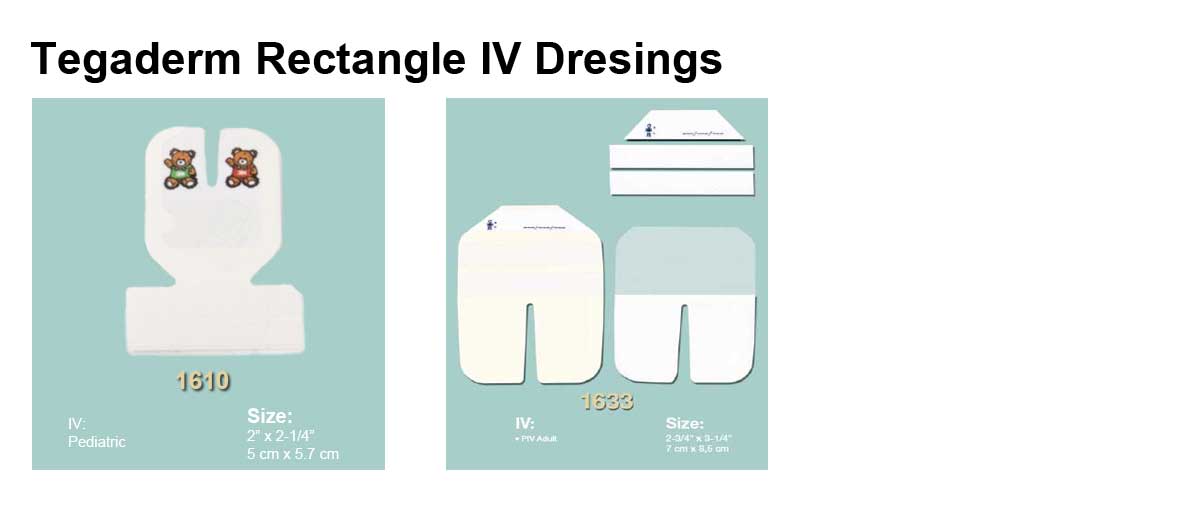

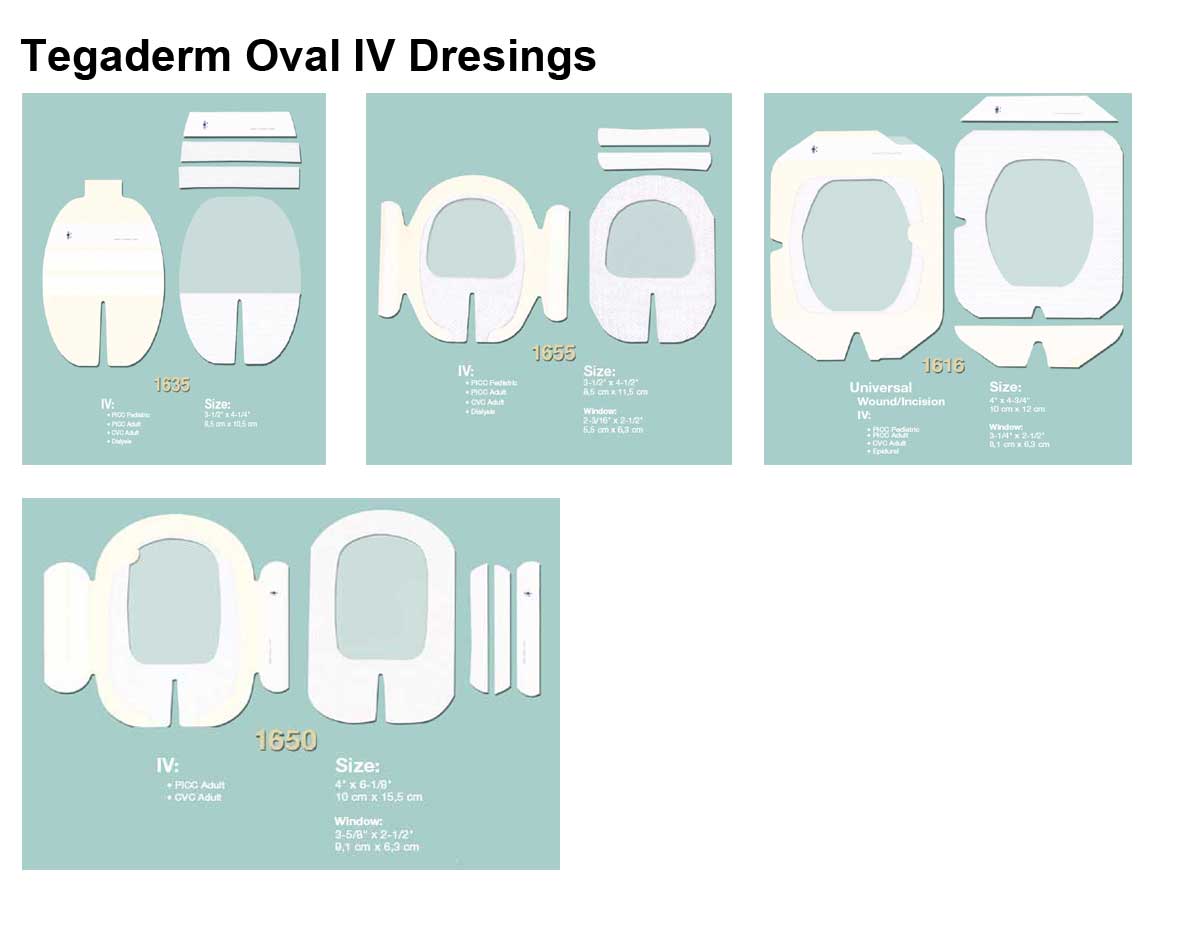
Applications
- To cover and protect catheter sites and wounds.
- Monitor catheter sites.
- Fix catheters to the body.
- To secure devices to the skin.
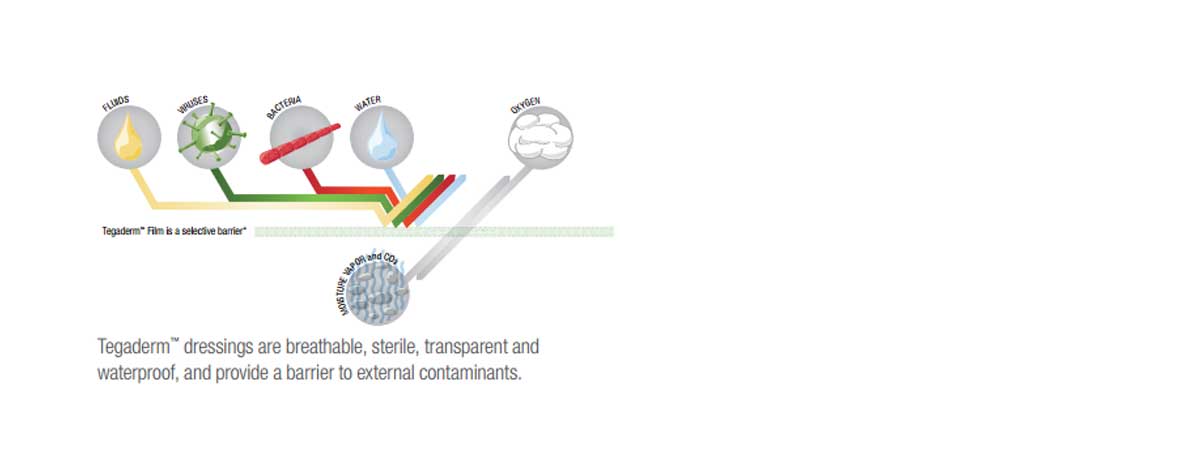
Features & Benefits
- Transparent to allow observation of the catheter site.
- Waterproof to protect against external contaminants.
- Notch design provides for a better seal around the catheter.
- Protects the puncture site.
- Dressings conform to the body and flex with skin for greater patient comfort.
- Reinforced notches reduce mechanical stress from heavy catheters.
- Sterile tape strips enhance securing of catheters or IV's.
Product Specifications
- Product Numbers: 1610, 1614, 1616, 1633, 1635, 1650, 1655.
- Size Options: 2 x 2.25 Inch, 2.375 x 2.75 Inch, 4 x 4.75 Inch, 2.75 x 3.25 Inch, 3.5 x 4.25 Inch, 4x 6.125 Inch, 3.5 x 4.5 Inch.
- Shape Options: Rectangle, Octagon and Oval.
- Color: Transparent.
- Application: I.V. Dressing.
- Material: Silicone coated paper liner, polyethylene-cellulose silicone carrier liner, acylate polymer, urethane polymer, polyester backing, rayon backing, polyethylene film.
- Contained by 2 paper liners.
- Solubility in water: Nil.
- Fire hazard: No.
- Manufacturer: 3M.
- UNSPSC Code: 42311527.
- HCPCS Code: A6257.

Manuals, Documents and Clinical Studies
 Sizes and Profiles contains a chart of the sizes and profiles.
Sizes and Profiles contains a chart of the sizes and profiles. Full Line of Tegaderm IV Products displays a profile and its corresponding item number.
Full Line of Tegaderm IV Products displays a profile and its corresponding item number. FAQ's for IV applications.
FAQ's for IV applications. Application Guide provides step-by-step instructions for properly applying.
Application Guide provides step-by-step instructions for properly applying. Clinical Studies offers citations to numerous studies of the advantages of this transparent dressing.
Clinical Studies offers citations to numerous studies of the advantages of this transparent dressing.- Khil, Myung?Seob, et al. "Electrospun nanofibrous polyurethane membrane as wound dressing."Journal of Biomedical Materials Research Part B: Applied Biomaterials67.2 (2003): 675-679.
- McCann, Beth. "Securing peripheral cannulae: Beth McCann discusses attributes and reports on an evaluation study."Paediatric Care15.5 (2003): 23-26.
- Haessler, Regina M. "Transparent IV vs. Traditional: An Inservice Evaluation."Journal of Infusion Nursing6.3 (1983): 169-170.
- Gilchrist, Thomas, and David Michael Healy. "Self-adhesive laminate." U.S. Patent No. 6,093,465. 25 Jul. 2000.

Frequently Asked Questions
What is the wear time for 3M Tegaderm IV Transparent Film Dressing?
The wear time for 3M Tegaderm IV Transparent Film Dressing is up to 7 days.
Is the 3M Tegaderm IV Transparent Film Dressing waterproof?
Yes, the 3M Tegaderm IV Transparent Film Dressing is waterproof.
What are the size options available for 3M Tegaderm IV Transparent Film Dressing?
The size options available are 2 x 2.25 Inch, 2.375 x 2.75 Inch, 4 x 4.75 Inch, 2.75 x 3.25 Inch, 3.5 x 4.25 Inch, 4x 6.125 Inch, and 3.5 x 4.5 Inch.
What materials are used in the 3M Tegaderm IV Transparent Film Dressing?
The materials used include silicone coated paper liner, polyethylene-cellulose silicone carrier liner, acylate polymer, urethane polymer, polyester backing, rayon backing, and polyethylene film.

Login and Registration Form